Polyurethane (PU) is the generic name for polymers possessing a urethane bond. A urethane bond is formed via an additional reaction between an isocyanate group and a compound possessing active hydrogen, such as a hydroxyl group.
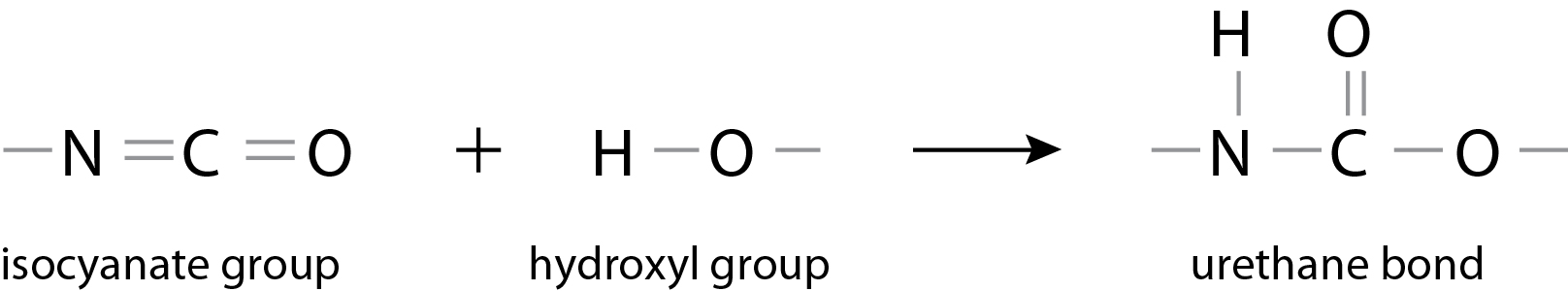
Isocyanate groups are extremely reactive and for this reason, this reaction proceeds without the addition of heat. A major feature is that, once reacted, an extremely stable structure is adopted. In other words, the external appearance of polyurethane takes on various forms, such as foamed products, films, elastomers, powders, liquids, and emulsions.
Compounds that include isocyanate groups include the following:
- 2,4 or 2,6-Toluene diisocyanate (a.k.a. tolylene diisocyanate or TDI)
- 4,4' or 2,4'-Methylene diphenyl diisocyanate (a.k.a. MDI)
- 1,6-Hexamethylene diisocyanate (a.k.a. HDI)
There are many more isocyanate compounds in addition to the above.
Compounds possessing two or more hydroxyl (OH) groups are called polyols and generally speaking, the following types are utilized.
- Polyetherpolyol
- Polyesterpolyol
- Polycarbonatepolyol
- Polycaprolactonepolyol
In addition, in place of hydroxyl groups, compounds such as carboxylic acids and amines, which possess active hydrogen, can be utilized in combination. On this account, when speaking of polyurethanes, one can say that there are limitless types.
Leveraging these features, polyurethanes are employed in a wide variety of areas, such as flexible foams, rigid foams, elastomers, adhesives, coatings, and binders.